Lecture 8 Matter, Atom and Elementary Particles
Matter
Any substance having mass and volume.
Based on physical conditions, such as temperature and pressure there are 5 states of matter
- Solid
- Liquid
- Gas
- Plasma- Presence of a significant portion of charged particles because of very high temperature
- Bose Einstein condensate- At absolute zero temperature atoms act as a single unit and the state is known as Bose Einstein condensate. This state of matter was predicted by Satyendra Nath Bose and Albert Einstein.
Atom
Smallest unit of matter consists of three subatomic particles(protons, neutrons, and electrons) with center known as the nucleus. The nucleus consists of the heavier atoms(protons and neutrons) while electrons orbit around the atomic nucleus in the outermost shells of an atom. John Dalton discovered the atomic theory(Atom) and It's nucleus was discovered by Ernest Rutherford.
In a neutral atom,
Number of electrons = Number of protons
Atomic Number- The number of protons in an atom.
Atomic Mass- The sum of the number of protons and the number of neutrons in an atom.
Isotopes- Various forms of an element that have the same number of protons, but a different number of neutrons such as 1H¹ and 1H².
Radioactive elements- isotopes/elements that have unstable nuclei are subject to radioactive decay.
Types of radioactive decay-
Alpha decay- α decay is a type of radioactive decay in which an atomic nucleus emits an alpha particle(helium nucleus) and transforms/decays into a different atomic nucleus.
Beta decay(electron capture)- β decay refers to transformation of a neutron into a proton, or a proton into a neutron. Beta decay either increases or decreases the atomic number of the nucleus by one.
Gamma decay- After alpha or beta decay nucleus get into an excited state. It can then decay to a lower energy state by emitting a gamma ray photon, in a process called gamma decay.
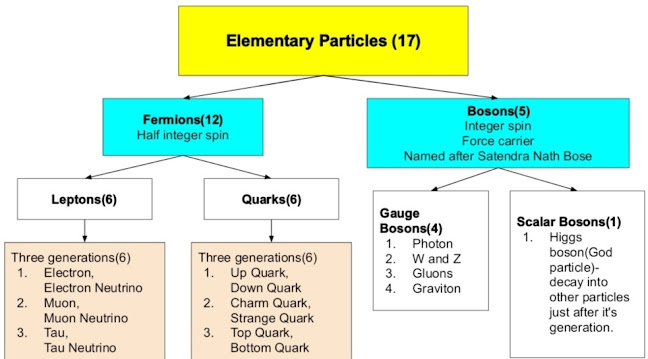
Standard Model of particle physics-
Four fundamental forces-
- Gravity- forces between two bodies having mass
- Electromagnetism- force because of electromagnetic field
- Weak interaction- interaction between subatomic particles that is responsible for the radioactive decay of atoms(nuclear fission and fusion)
- Strong interaction- it confines quarks into protons, neutrons and other hadron particles. The strong interaction also binds neutrons and protons to create atomic nuclei thus also called nuclear force.
Strength of forces
Gravity< Weak< Electromagnetic< Strong
Standard Model of particle physics- It describe three fundamental forces except gravity and give the classification of elementary particles(smallest unit of atom that is not composed of other particles).
Nutrino(Ghost particles)- tiny subatomic particles with negligible mass and charge neutral often called ghost particles because they barely interact with anything else.Neutrinos are omnipresent in nature and every second billions of them pass through our bodies without us ever noticing.
Despite how common they are, neutrinos are extremely difficult to detect, due to their low mass and lack of electric charge. Countries around the world deployed various measure to detect neutrino. India-based Neutrino Observatory(INO) is a particle physics research project to study atmospheric neutrinos in a deep cave under INO Peak near Theni, Tamil Nadu, India.
Quantum Mechanics- The discovery of the elementary particles gave rise to quantum theory. It is the study of matter and its interactions with energy on the scale of atomic and subatomic particles. It helps to understand the universe at small scale.
It explains how extremely small objects simultaneously have the characteristics of both particles and waves(wave-particle duality).


Comments
Post a Comment